Anyone who uses solvents to extract Cannabinoids on a large scale will tell you that the solvents are expensive. Not only is it cost-effective to recover and reuse the solvents, it is wise to use only as much solvent as is necessary, and not more. Using excess solvent lengthens the purge time and reduces the capacity of your equipment.
Of course, it is also true that the more solvent you use the higher your total yield. So, a balance must be struck between using more solvent to improve yield, and using less solvent to increase productivity. On both ends of this spectrum are diminishing returns, and the cost-benefit analysis is dependent on where priorities are placed.
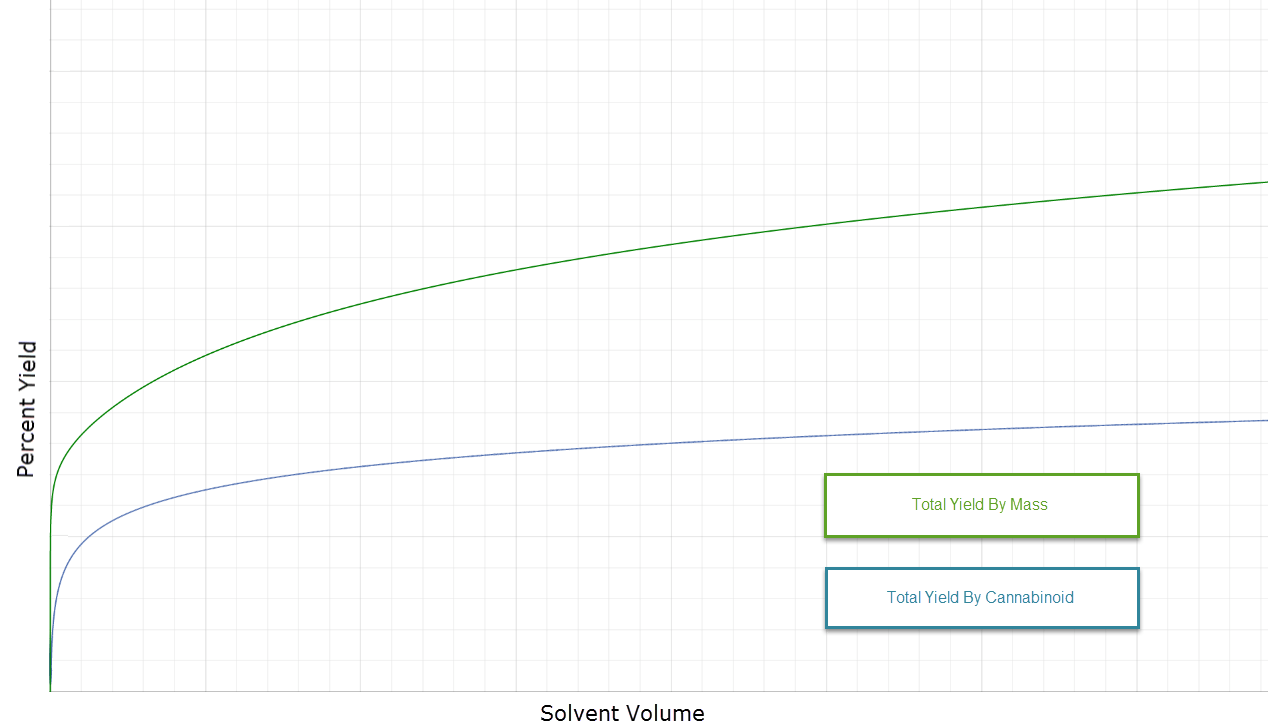
What many may not realize is that the solvent-to-substrate ratio has implications not only to productivity and yield, but also to purity of final product. Consider the posted graph to illustrate the concept.
The green line indicates the total yield by mass. That is: how much final product do you get as a percentage of your starting material? We must recognize that our final product is not 100% pure cannabinoid… the mass of the cannabinoids is less than the total mass of the final product. As such, the blue line indicates the amount of cannabinoids recovered as a percentage of the total starting material.
What these data indicate is that the more solvent you use the more material you capture, but the lower purity of final product. As solvent quantity increases, the amount of cannabinoid capture depreciates faster than does the amount of impurity capture.
By no means is this an argument in favor of using less solvent. There is certainly an argument for higher yield even if it sacrifices potency a little. It’s a balancing act. As a producer, you should ask yourself what you desire from your extraction process. On what vertical line of this graph do you want your extraction outcome?
Note: The graph above is not to scale and depends on a number of factors including what type of solvent is being used. If you want to find out how your solvent behaves so as to maximize your outcomes, ask your laboratory to help you conduct a similar experiment.